Abstract
Background: Elevated serum levels of the thymus and activation-regulated chemokine/CCL17 (TARC) reflect tumor burden in classical HL at diagnosis and their reduction during treatment can predict prognosis. Interim PET, performed after 2 cycles of ABVD (PET2), proved to be highly effective in identifying advanced-stage HL patients at risk of failure by continuing ABVD (Gallamini A et al. J Clin Oncol 2007;25:3746), whereas their prognosis could be improved by shifting to BEACOPP escalated (Gallamini A et al. Haematologica 2017;102s: S413). Nevertheless, a not negligible fraction of patients (13%-15%) despite a negative PET2 is reported to experience failure with ABVD, and only 60% of patients are expected to be salvaged by BEACOPP escalated. Therefore, the evaluation of biomarkers like serum TARC levels, an easy to perform and relatively inexpensive procedure, could be useful to assess risk of disease progression in patients treated with a PET-adapted strategy. The aim of this study was to evaluate the correlation of PFS and early serum TARC levels reduction in patients with advanced-stage HL treated according to HD0607 PET-driven trial.
Methods
HD0607 trial was a prospective open label phase II study treating, from June 2008 to June 2014, 782 newly diagnosed advanced-stage HL patients with 2 cycles of ABVD, followed by PET2; PET2-negative patients continued with 4 cycles ABVD and PET2-positive patients were randomized to 4 cycles BEACOPP escalated followed by 4 cycles BEACOPP standard with or without Rituximab. Serum TARC levels were measured by commercially available ELISA test kits (R&D System, Minneapolis, USA) on frozen sera of patients at baseline and after 2 ABVD cycles (T2). The value of 800 pg/mL, representing the 99th centile of TARC distribution in a group of 156 independent healthy subjects, was considered as cut-off value discriminating between normal and abnormal TARC values. The Wilcoxon Mann Whitney test was used to analyze the association between median TARC values and PET2 results or treatment outcome. Kaplan-Meyer curves and log-rank test were used to assess differences in PFS according to TARC levels. Cox model was used for multivariate analysis.
Results
The serum TARC levels were assessed in 243 patients at baseline and after 2 ABVD cycles. Main patient characteristics were as follows: males/females: 122/121, median age: 31 years (range, 16-60), B symptoms: 86%, bulky disease (>5cm): 59%, stage IIB: 43%, stage III: 31%, stage IV: 26%, IPS>3: 14%. Median (IQ range) TARC values at baseline and after 2 ABVD were: 37855 (13990-73312) and 444 (277-723) pg/mL, respectively. A positive PET2 was recorded in 47/243 (19%) patients and 46/243 (19%)patients experienced disease failure. Median (IQ range) T2 values were significantly higher in PET2-positive patients compared to their counterpart (703, IQ 389-2517 vs 424, IQ 250-656; p<.001), and in patients with progression or relapse compared to those in continuous CR (580, IQ 308-1297 vs 429, IQ 277-661; p=.01). With the selected cut-off of 800 pg/mL, the sensitivity and specificity of T2 in predicting PET2 results and treatment outcome were 0.43, 0.82 and 0.41, 0.82, respectively.
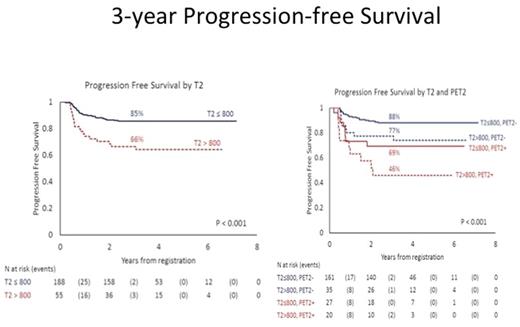
As shown in Figure 1, after a median follow-up of 3.6 years, the 3-year PFS for patients with T2 ≤ vs >800 pg/mL was significantly different: 85% vs 66% (p<.001), and T2>800 pg/mL was able to identify patients with a worse prognosis also among those with a negative PET2 (3-year PFS: 77% vs 88%; p=.03). In univariate and multivariate analysis T2 > 800 pg/mL, positive PET2 and IPS>3 significantly affected PFS. T2 was a significant independent predictor of outcome in both the whole study population (HR 2.26; 95% CI :1.22-4.20) and in the PET2 negative patients (HR 2.37; 95% CI: 1.06-5.26).
Conclusions
Early reduction of serum TARC levels, after 2 ABVD cycles, are associated with a favorable outcome in patients treated with a PET-driven strategy. Evaluation of T2 may help identifying those patients at risk of treatment failure despite a negative PET2.
Viviani: Takeda Italia: Speakers Bureau; Takeda International: Research Funding. Zanotti: Deciphera: Consultancy. Corradini: Sanofi: Honoraria; Roche: Honoraria; Gilead: Honoraria; Takeda: Honoraria; Novartis: Honoraria; Janssen: Honoraria; Celgene: Honoraria; Amgen: Honoraria. Rambaldi: Novartis, Amgen, Celgene, Sanofi: Other: Travel, Accomodations, Expenses; Novartis, Roche/Genentech, Amgen, Italfarmaco: Consultancy.
Author notes
Asterisk with author names denotes non-ASH members.